Organic acids can be defined as organic compounds with acidic properties associated with their carboxyl group ‘-COOH’. It is of high importance to understand the different chemical properties and actions of these components to choose the most optimal solution for the challenge in place. Although MCFA can be classified in the category of organic acids, their chemical structure differs from standard organic acids. In this way, MCFA continue to prove their versatility in raising healthy animals by offering a broad range of beneficial effects on feed and animal level.
Antibacterial? pKa and HLB
Acids can be divided in several subgroups depending on their molecular structure and chemical characteristics (Figure 1). Two properties, the pKa and HLB value, are major factors determining the antibacterial and pH reducing capability of the different acids.
Each acid has its own pKa value which is equal to the pH at which 50% of the acid appears in its undissociated form and 50% in its dissociated form. If the environmental pH is lower than the fixed pKa value, the acid will shift towards its undissociated form. When the environmental pH is higher than the pKa value of the acid, the acid will shift towards its dissociated form. In this way, acids such as formic (pKa = 3.75), lactic (pKa = 3.86), fumaric (pKa = 3.02) and citric acid (pKa = 3.10) are typical pH-reducing acids while propionic acid (pKa = 4.88), butyric acid (pKa = 4.82), acetic acid (pKa = 4.76) and MCFA (pKa = 5) will remain more undissociated enabling these acids to approach negatively charged bacteria and act antibacterial, rather than reducing the pH.

Even within the group of antimicrobial acids there is a large difference in effectiveness. This can be mainly explained by differences in the hydrophilic lipophilic balance (HLB) which is an expression of the ratio of hydrophilic over hydrophobic parts in a molecule. A bacterial cell membrane consists out of phospholipids which are amphiphilic, meaning that they contain a hydrophilic and a lipophilic part. For an acid to be an efficient destabilizer of the bacterial cell membrane, the HLB of the acid molecule must be similar to that of the bacterial cell membrane. Especially MCFA show this great similarity on HLB resulting in the highest antibacterial effect (Table 1).
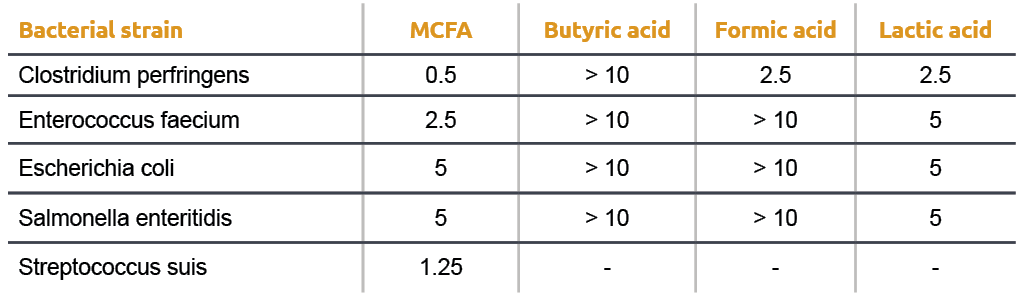
Obviously many other properties such as physical state, smell and taste are also important factors determining application, processing and actions of the different acids.
Mode of action
Organic acids have different actions depending on their specific chemical characteristics. Regulation of the acidity is probably the most well-known effect of organic acids. Acids such as formic, fumaric and lactic acid will typically dissociate when coming in contact with water, resulting in a reduction of the pH value. In this way, organic acids with a low pKa value might exert a bacteriostatic effect in the animal, in drinking water or in feeds. A pH reduction in the stomach also leads to better protein digestion.
A second group of acids has direct antibacterial effects. This group is particularly important with regard to the reduction of antibiotics and typically entails acids with a relatively high pKa value. These acids will remain undissociated, destabilize the microbial cell membrane and disturb the bacterial metabolism by reducing the intracellular pH and intercalation with DNA. MCFA are outperforming any other type of acids in this respect due to their high pKa value and optimal HLB. Furthermore, MCFA act antibacterial directly at stomach level in comparison to coated acids and are selective towards Enterobacteriaceae leaving the beneficial Lactobacilli untouched.
Other modes of action which are more assignable to specific acids include improvements of gut morphology (e.g. longer villi, higher villi:cryp ratio…), reducing pathogen virulence (e.g. Salmonella), improving immunity (e.g. longevity neutrophils, decreased inflammation…), antifungal and antiviral properties. Table 2 gives a comprehensive overview on the effects of different acids on this level. Lately the effects of MCFA against different emerging viral diseases like PRRS, PED and ASF have been scientifically proven by independent research institutes and universities.

Applications and solutions
Depending on the chemical characteristics and actions, it’s possible to define the most suited solutions for different challenges. For very specific applications, simple organic acids might still be the most desired solution. On the other hand, based on the versatility of actions and strengths, MCFA offer a broader range of benefits. By applying expertise in optimizing MCFA based solutions, Agrimprove has developed specific solutions focused on different challenges. In this way, Agrimprove has recently launched the 100% MCFA-based solution FeedLock to mitigate the spreading of enveloped viruses via feed and Eubisol, a synergistic mixture of organic acids and MCFA to improve animal health in a flexible way via drinking water.
