By Josep Garcia-Sirera (Toxin Binder Product Manager)
All animal species are affected by gram-negative bacteria. Under normal circumstances, the bacteria are present in the gastrointestinal tract in equilibrium with other microorganisms. However, in situations of bacterial challenge—induced by stress, immunosuppression, etc.—the equilibrium can alter and cause an excess of gram-negative bacteria. During these imbalances, endotoxins are produced and released in the animal.
It is under circumstances of a high gram-negative to gram-positive bacteria ratio that endotoxins, also known as lipopolysaccharides (LPS), can become a problem. An excess of LPS happens when there is a high turnover of gram-negative bacteria or a rapid destruction of gram-negative bacteria due to the use of antibiotics. An inflammatory pyrogen, lipopolysaccharides can trigger detrimental effects by stimulating an inflammatory reaction in the gastrointestinal tract and once absorbed, at other sites in the animal.
Overview of the endotoxin’s structure
What makes gram-negative bacteria differ from gram-positive bacteria is the structure of the cell envelope. Gram-positive bacteria have a cytoplasmic and a peptidoglycan outer membrane. In addition to these layers, gram-negative bacteria have an addion outer layer. The outer layer contains phospholipids, proteins, and LPS. This LPS consists of three elements: Lipid A (a hydrophobic component that is toxigenic), a core (an oligosaccharide), and O-antigen (hydrophilic component projecting into the extracellular space) (Figure 1).
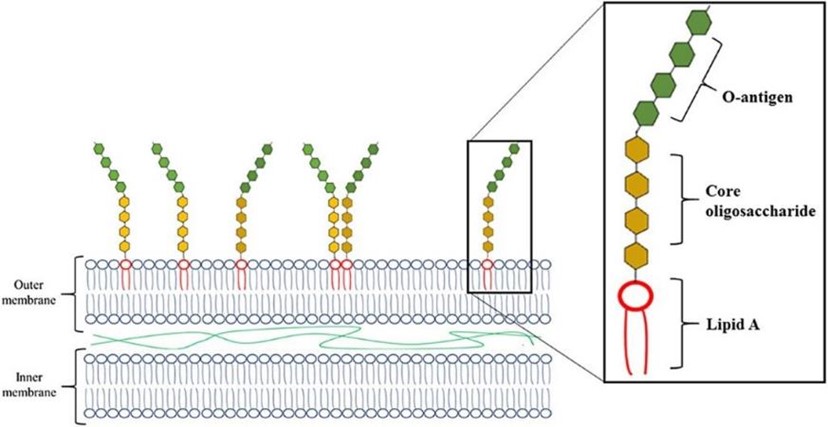
Understanding endotoxin behavior
The mechanism of action is complex. Put simply, a lipopoly¬saccharide binds to a lipid-binding protein (LBP) to create the LPS/LBP complex that will eventually bind with Toll-like receptor-4 (TLR4). This triggers the signaling cascade for macrophage/endothelial cells to secrete pro-inflammatory cytokines and nitric oxide that lead to the characteristic “en-dotoxic shock” (Figure 2).
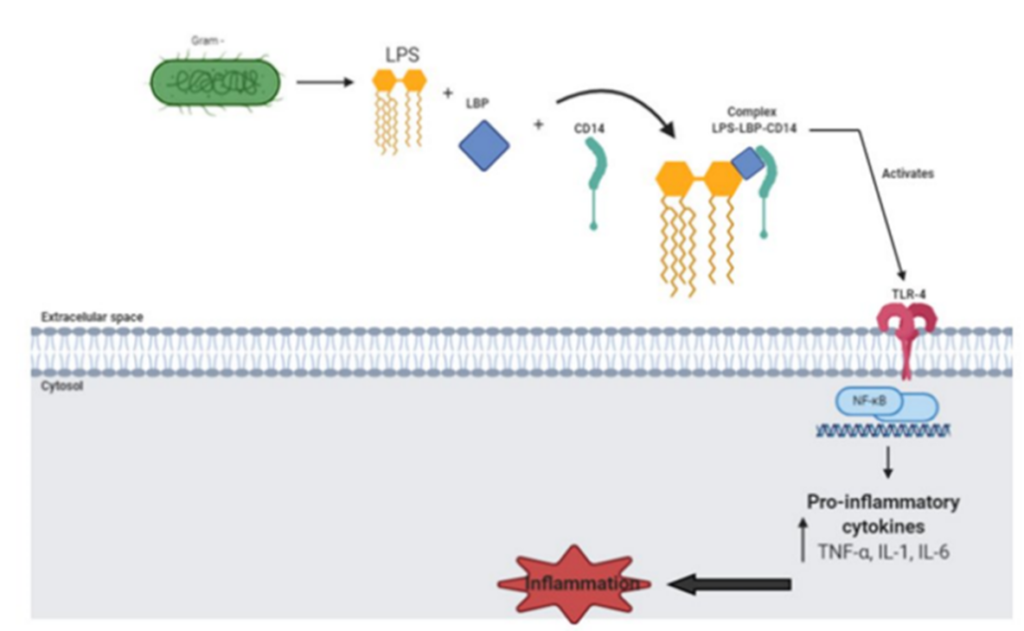
Presentation of endotoxic shock
Present in dust, feed, and feces, endotoxins are on every type of farm. However, different production species are not affected equally when it comes to an LPS. While it is well documented that all animals have receptors for LPS, the way an animal reacts to the presence of LPS varies greatly.
Poultry
Of production animals studied, poultry is the least affected by LPS. Their sensitivity to LPS action is much lower than swine or ruminants. The theory for this is due to differences in the TLR4 receptor. Utilizing the lock and key concept, the TLR4 activation site responsible for endotoxin action is slightly different from mammals, this could partly explain the lower effects of LPS in poultry. Though there is little research, some possible signs of endotoxins in poultry are fatty liver, reduced feed intake, wet litter, and decreased performance; be it laying rate and shell quality, growth, or reproduction.
Ruminants
In ruminants, particularly dairy cows, LPS is extensively described as a factor of the subacute ruminal acidosis (SARA) pathological effect. Consumption of a high concentrate diet by dairy animals enhances the production of organic acids and results in lower pH values in the rumen and intestine. This decrease in pH affects, among other things, the equilibrium of the microbes in the rumen. The decline in pH perturbs the balance of the microbial population in the rumen to cause a substantial release of free endotoxin from gram-negative bacteria. During SARA, LPS is greatly increased in both the ruminal fluid and blood, triggering the pathologies associated with SARA: metritis, laminitis, fatty liver, retained placenta, etc. Different studies have shown levels of LPS in ruminal fluid during SARA to reach higher than 150,000 EU/mL.
Swine
Similar to other species, LPS’s effect on swine production comes from the inflammatory reaction endotoxins cause.
First, the gastrointestinal barrier alters due to the inflammatory response. A study on the effects of endotoxins on the morphology of the gastrointestinal gut barrier showed that LPS decreased the height and area of intestinal villi, increased the width of the villi, and increased the depth and width of the intestinal glands. The authors concluded these effects contribute to decreased intestinal nutrient absorption and increased co-infection with other pathogens, leading to post-weaning diarrhea syndrome.
Second, initiated by exposure to endotoxins, fever is mediated through the action of IL-6. There are other symptoms mediated through the action of the different cytokines but all are related to the inflammatory response. As a result of inflammation, endotoxemia leads to a feverish state that results in reduced feed intake.
Finally, with the activation of the inflammatory system, there is a nutrient expense. This results in a lower feed conversion rate (FCR) and decreased growth performance.
Managing endotoxins
In general, strategies to control endotoxin contamination in animals are designed to limit bacterial contamination. These strategies include biosecurity, prebiotics, probiotics, improved nutrient digestibility, etc. Other strategies include vaccination (expensive and currently only applied to humans), immunomodulation (to reduce inflammation), and the use of a toxin binder scientifically proven to target endotoxins (the most practical approach).
Since gram-negative bacteria are always present, the use of a scientifically supported endotoxin binder to reduce the absorption of endotoxins is a practical solution for many operations. Toxin binders are widely used to control other toxins, such as mycotoxins, and work by binding the toxin into a form too complex and large to be absorbed into the blood system. The complex is then eliminated in the feces. Most mycotoxin binders are hydrophilic molecules (e.g., bentonites and aluminosilicates) efficient at capturing polar molecules such as Aflatoxin. The capacity of these traditional mycotoxin binders to capture more lipophilic-like molecules such as LPS, zearalenone, or DON is questionable.
In vitro endotoxin binding
Agrimprove’s toxin binder has been extensively tested under in vitro conditions for its capacity to bind endotoxins at both low and high concentrations. In addition, an in vitro study using HPLC (High Precision Liquid Chromatography) to understand the adsorption of endotoxin by the toxin binder and a commercial endotoxin binder was performed. To mimic the conditions present in the gastrointestinal tract of animals, the in vitro test was performed using a pH of 5.0 for adsorption and 6.5 for desorption. The results of this in vitro test have been corroborated on several occasions (Graph 1).
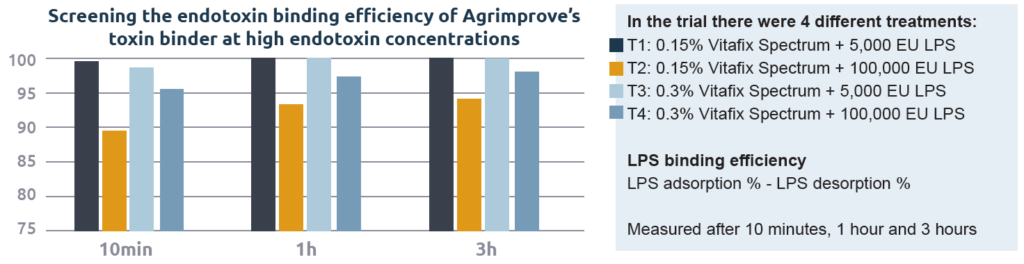
In vivo endotoxin binding
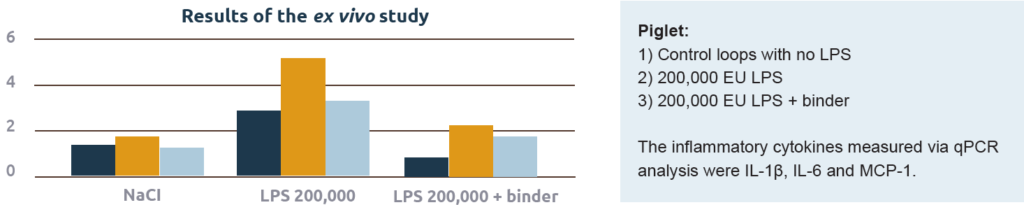
These in vitro results demonstrate the potential capacity of a binder to capture toxins. Still, the fact remains that a product needs to prove effective in the animal. A recent ex vivo study by the University of Ghent effectively proved the capacity of a toxin binder to capture endotoxins in the intestine of piglets (Graph 2). In the study, the authors compared the effect on the production of cytokines by injection of endotoxins with or without the addition of the toxin binder. The model was intestinal loops of live piglets (Figure 3).
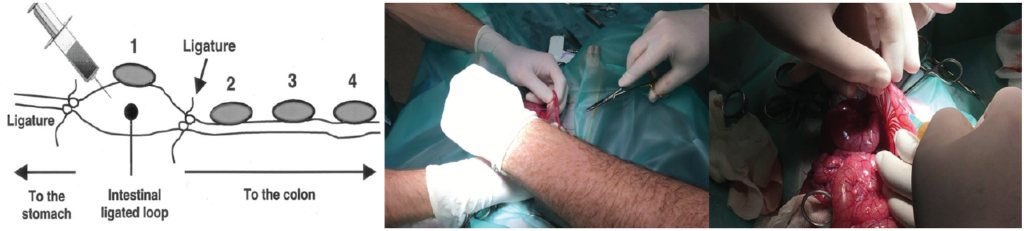
injected directly in the intestinal loop and a biopt of each look was taken to determine endotoxin binding potential
Endotoxins have a wide variety of effects on livestock, including depressed feed intake, fatty liver, and loose feces to name a few. Different means to control endotoxins have been tested but most of them are too expensive to be considered in animal production. A cost-effective method is the use of toxin binders to capture endotoxins in the gastrointestinal tract and prevent them from entering the blood system. This binding approach stops endotoxins, not only in the gastrointestinal tract and bloodstream but also from going airborne in the dust from feces.
Your Agrimprove expert
THIS MIGHT ALSO INTEREST YOU
Ready for improvement? Try one of our suggested solutions with a proven added value.



