Written by Kobe Lannoo, Global lead swine at Agrifirm
Managing intestinal health of newly weaned piglets remains a challenge; advances in our understanding of the problem are offset by ever tightening regulations. Competitive exclusion is one tool which is not known to affect resistance genes in bacteria but which can provide interesting benefits in practice.
At the 2023 Think Piglet conference, it was stated that E. coli is the primary cause of diarrhea in nursery piglets responsible for one in every three cases (Peters). E. coli is omnipresent and commonly found in the intestinal tract of animals. When E. coli becomes dominant, though, it can induce disease which ultimately can be fatal. It does so by producing toxins that bind to epithelial cells eliciting the secretion of electrolytes while blocking sodium absorption. This results in massive fluid loss from the epithelium into the intestinal lumen which shows itself as diarrhea (Moeser et al., 2007).
Strategies for preventing E. coli diarrhea outbreaks are multiple, including:
- Directly inhibit E. coli, for example, by using medium-chain fatty acids and/or organic acids
- Prevent E. coli from binding to the enterocytes, avoiding biofilm formation
- Intercept the toxins produced by E. coli, e.g., by using toxin binders
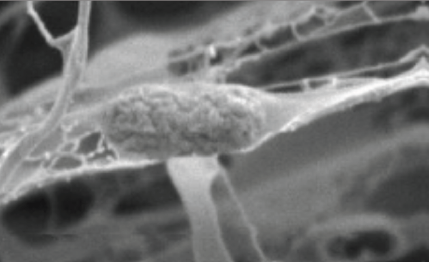
The strategy which today does not get a lot of attention is to prevent it from binding to enterocytes, more elegantly called ‘competitive exclusion’. The idea behind this competitive exclusion is that E. coli needs to adhere to enterocytes in order to form a biofilm. Mannose-directed adhesion is a very common described binding ability of bacteria. By feeding mannose as part of an indigestible structure, the E. coli may well bind to this indigestible structure instead of to enterocytes (Figure 1). Instead of forming a harmful biofilm, though, E. coli bound to such indigestible structures are excreted with the feces. This results in lower levels of E. coli in the piglet, and thus less risk of causing disease.
To verify this mode of action, Agrimprove carried out a field test with its Vitafibra concept, the outcome of that test was recently published (Tanghe et al., 2023). Vitafibra is composed of two bioactives, including a mannose-based competitive exclusion fiber, as well as a prebiotic fiber. Newly weaned piglets (n=200, housed in pens of 10 and weaned at 23 days of age) were fed either a control diet, or a diet supplemented with 0.2% Vitafibra. Two weeks after weaning, 20 piglets (1 per pen) were sacrificed and intestinal contents were analyzed.
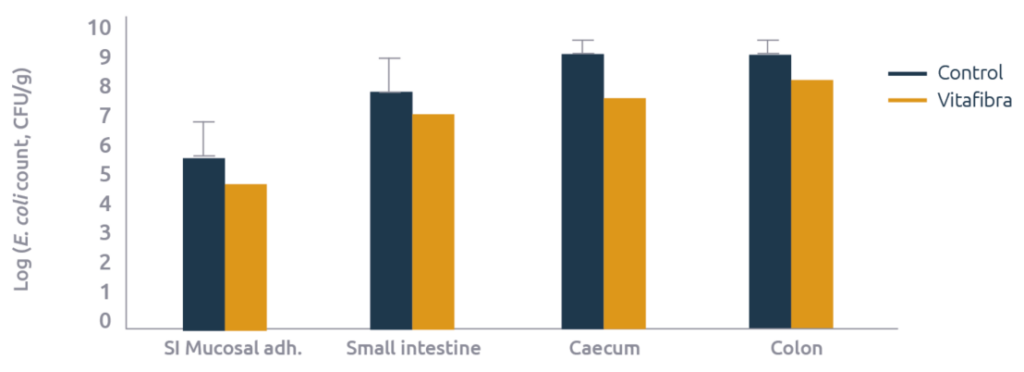
E. coli was enumarated in the lumen of the small intestines (25% before the caecum), caecum, and colon. At the same location in the small intestine, mucosal scrapings were also collected and E. coli were enumarated in this scraping as a measure of biofilm formation. For each E. coli measurement, a roughly 10-fold reduction in E. coli count was observed in the animals fed Vitafibra as compared to the controls (Figure 2).
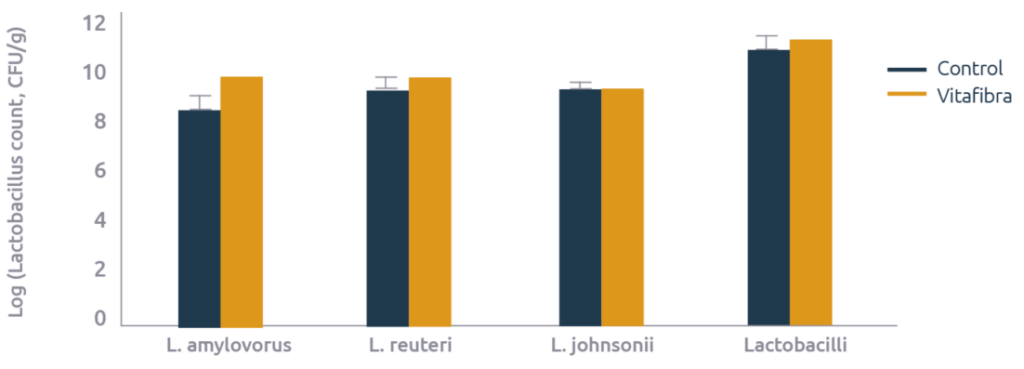
In addition to E. coli, microbiota was further characterized which revealed that the reduction in E. coli in the small intestines was offset by an increase in Lactobacilli: L. amylovorus increased 15 fold, L. reuteri 4 fold, and the total Lactobacilli count increased numerically by 1.6 fold (Figure 3).
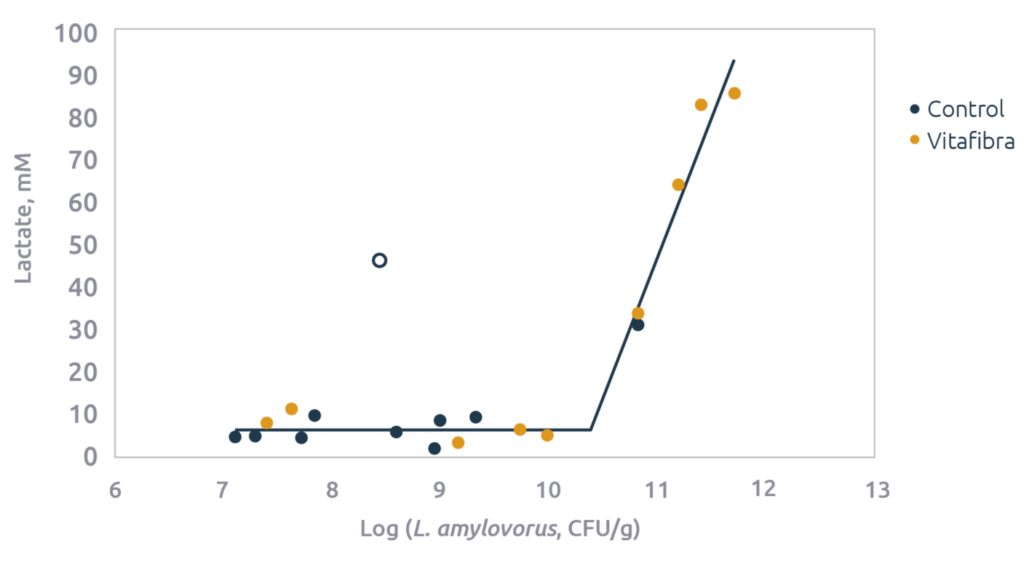
In line with the increase in Lactobacilli a strong increase in lactic acid levels were observed (2.6 fold). In fact, lactic acid levels correlated quite strongly with levels of the different Lactobacillus measurements (Figure 4). Lactobacilli actually use lactic acid as a mean to improve their environment; Lactobacilli themselves are quite acid tolerant, but many pathogenic bacteria, including E. coli, are not. Thus, by increasing lactic acid and consequently lowering pH they reduce E. coli while allowing themselves to prolificate. L. amylovorus does so by fermenting starch (hence its name) into lactic acid. This strategy is actually a win-win for both the Lactobacilli as well as the host as it also improves intestinal health.
Last but not least, myeloperoxidase was measured as a biomarker of neutrophil activation by invading E.
coli. Myeloperoxidase was half (10.4 vs. 20.2±3.5 ng/g feces) in the animals receiving Vitafibra, confirming
that the feeding strategy and the resulting shifts in microbiota reduced inflammation in the intestinal tract.
In conclusion, Vitafibra decreased E. coli counts by a factor 10 throughout the intestinal tract which apparently allowed Lactobacilli to thrive in the small intestines as their numbers increased, as did the concentration of lactic acid. This resulted in a significant reduction in intestinal inflammation. Consequently, Vitafibra is an interesting tool for fighting E. coli diarrhea in newly weaned piglets.