By Marta Bruguera Bonfill, Gobal technical lead swine
Endotoxins are toxic compounds that are released from the outer membrane of the cell wall of gram-negative bacteria during their lysis. Gram-negative bacteria can cause damage by themselves, but also by the endotoxins they release. The intestinal barrier is the organism’s first line of defense, protecting it from the major source of endotoxins, the intestinal contents. Piglets at weaning are the most vulnerable animals to endotoxins, because of their digestive immaturity and the multiple stress factors that compromise the functionality of their intestinal barrier and the balance of their intestinal microbiota.
The piglet’s organism recognizes endotoxins as a foreign body that triggers an inflammatory response that, in the least of cases, reduces piglet growth and performance due to the utilization of nutrients to meet the needs of the immune system. In more severe cases, the immune response to endotoxins degrades performance and leads to fever, systemic inflammation, reduced feed intake, increased intestinal permeability, and digestive disorders. These symptoms occur when intestinal permeability increases, allowing endotoxins to enter the systemic and lymphatic circulation. In addition, when the body’s detoxification capacity is exceeded, the piglet dies from endotoxemia, shock and multi-organ failure.
A wide range of indirect measures can be taken to counteract the effects of endotoxins. These include strategies to reduce the presence of gram-negative bacteria and stress, and to enhance intestinal integrity and maturity in piglets. However, at present, while methods of direct control of the toxic effects of endotoxins exist for humans, only a few strategies are economically feasible for swine.
Weaning is the most critical time
The most critical time for the piglet is weaning, when multiple stress factors compromise its performance and health. The separation of the piglet from its mother, and the change from a liquid diet (milk) to a solid diet (feed), are sudden changes that generate great stress. Other changes, such as transport, mixing of animals, establishment of new social hierarchies, change of environment, management, and adaptation to new feeders and drinkers, add to the stress. Moreover, the implementation of vaccination programs and the presence of pathogens make weaning the period of greatest demand and, in turn, of greatest risk for the piglet.
At the time of weaning, the piglet’s gastrointestinal tract (GIT) is not yet fully developed, and it has a limited capacity for digesting and absorbing nutrients. This is aggravated by the reduced water and feed consumption after weaning, which induces changes in the morphology and functionality of the piglet’s GIT. So, protecting weaned piglets from endotoxins is crucial during this phase of their lives.
Intestinal microbiota
The intestinal microbiota play a determining role in specific functions in nutrient metabolism, immunity, and in preserving the integrity of the intestinal mucosal barrier. Therefore, it is important to maintain a balance here.
The physicochemical conditions vary in different parts of the GIT, and so the microbial population living in each area is also different. The small and large intestines are areas of preferential colonization by opportunistic bacterial pathogens. However, further studies are needed to fully understand the compositions of the microbiota throughout the GIT and the mechanisms of dysbiosis for its prevention. In general, we can say that it is important to maintain a balance between gram-positive and gram-negative bacteria. Weaning stress, together with the immaturity of the piglet’s GIT, reduced consumption and a change in diet, are associated with alterations in the balance of the intestinal microbiota, diarrhea, greater susceptibility to enteric infections, the use of antibiotics, and reduced performance. This intestinal dysbiosis can be correlated with a large proliferation of gram-negative bacteria such as Enterobacteriaceae (for example, E. Coli, Salmonella spp.) that displace the beneficial intestinal microbiota (such as Lactobacillus, among others), causing diseases such as colibacillosis or salmonellosis.
Gram-negative bacteria and endotoxins
Gram-negative bacteria can cause damage by themselves, but also by the endotoxins they release. Unlike gram-positive bacteria, which do not have an outer membrane, gram-negative bacteria have a characteristic cell envelope structure that protects them. Gram-negative bacteria are surrounded by the inner cytoplasmic membrane, a thin cell wall of peptidoglycans and another outer membrane that contains phospholipids, membrane bound proteins and lipopolysaccharides (LPS) (see Figure 1).
Free LPS are called endotoxins due to their toxicity and ability to induce an immune response in the animal when they enter the systemic circulation (although both terms are used interchangeably in the literature).
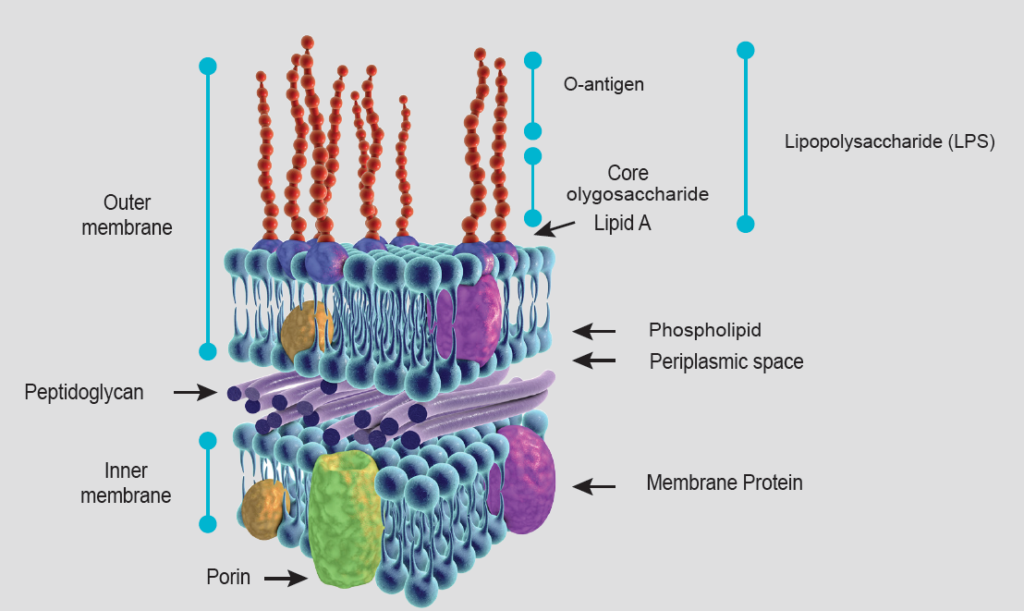
LPS consist of 3 structural elements: lipid A, the oligosaccharide core, and the O-antigen (Figure 1). Lipid A anchors LPS to the outer membrane of the bacteria (hydrophobic component). It is responsible for most of the toxicity caused by endotoxins, and its structure and toxicity can vary between bacterial species.
The O-antigen (also called O-specific antigen) is a chain of oligosaccharides of variable composition depending on the species and strain of gram-negative bacteria and is found in the extracellular part (hydrophilic component). It is highly immunogenic and stimulates the production of antibodies, although these do not protect against the toxic effects of LPS since this part is not pathogenic.
LPS release and sources
LPS is released from gram-negative bacteria during cell lysis. This occurs during cell replication and death; and contact of the bacteria with water. Bacterial death can occur by the action of the immune system or when we use antibiotics, especially β-lactams. The rate of release of LPS varies between different species and strains of bacteria. As endotoxins come from gram-negative bacteria, they are ubiquitous in the environment. They can be found in water, feed, soil and air, but the main source is in the GIT and feces.
In the GIT, endotoxins are present especially in the large intestine, mostly from gram-negative bacteria of the Enterobacteriaceae family, such as E. Coli and Salmonella spp. They can be excreted in feces, contaminate dust particles from feces and then be transmitted by aerosols, which, when inhaled by the animals, can cause inflammation and respiratory or gastrointestinal diseases.
The intestinal barrier is the organism’s first line of defense, which protects it from the largest source of endotoxins, the intestinal content. Piglets at weaning are the most vulnerable animals to endotoxins. This is due to their digestive immaturity and the multiple stress factors that compromise the functionality of their intestinal barrier and the balance of their intestinal microbiota.
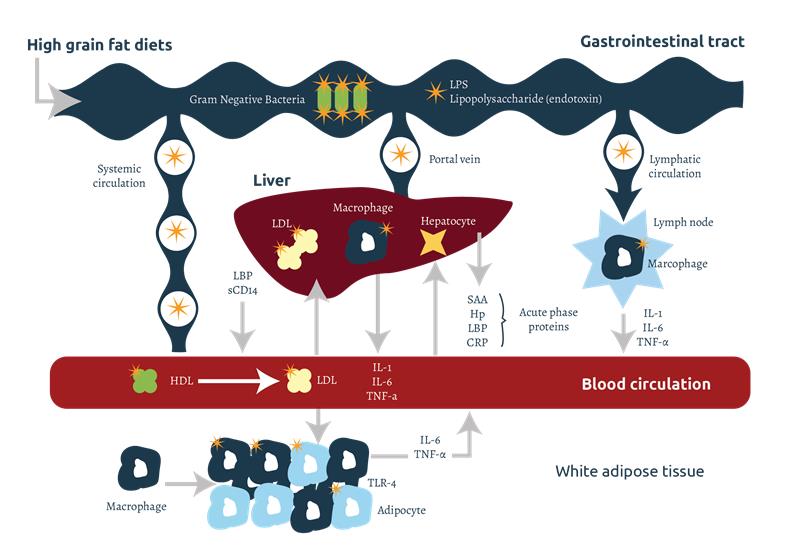
Endotoxins released at the intestinal level can pass into the systemic and lymphatic circulation by transcellular transport, through endocytosis mediated by receptors present in the epithelial cells. During the intestinal digestion of dietary fats, the formation of micelles also facilitates the permeability of LPS (see Figure 2).
Interaction between endotoxins and other pathogenic factors
Other factors that can increase the absorption of endotoxins from the GIT into the bloodstream are: oxygen free radicals (ROS) and mycotoxins.
Normally, the cells of the animal body generate ROS as a result of metabolic processes. ROS are highly reactive compounds that can induce the oxidation of intra- and extracellular molecules that directly affect cell viability and function. In an ideal situation, a balance is established between oxidation and antioxidant mechanisms, which safeguards cell integrity and health. However, during stressful periods such as weaning, thermal stress, presence of vomitoxin (DON), and other stressors, the production of ROS can exceed the body’s natural capacity to detoxify them, which generates oxidative stress.
The GIT is affected greatly by oxidative stress. High ROS levels are capable of oxidizing fundamental cellular components (proteins, DNA, lipids, carbohydrates), inducing apoptosis of intestinal cells and resulting in a lower absorption capacity (shorter villi). Tight junctions between enterocytes prevent the passage of macro-molecules from the intestinal lumen into the bloodstream. In situations of oxidative stress, ROS cause lower expression of the genes that code for tight junction proteins. As a result, tight junctions dissociate, increasing intestinal permeability and allowing paracellular transport (between enterocytes) of pathogens, LPS and toxins. In these cases, a large passage of LPS into the piglet’s bloodstream and lymphatic system will trigger an immune response, which generates inflammation, increased intestinal permeability, digestive disorders, fever, reduced consumption, and lower performance. If the body’s detoxification capacity is exceeded, LPS can cause systemic inflammation, septic shock, endotoxemia, multi-organ failure, and the animal’s death.
Endotoxins metabolic pathway
Endotoxins released into the body bind to carrier proteins present in cells and body fluids, which facilitate their transport, recognition and degradation. There are several types of carrier proteins, such as sCD14 and lipoproteins, although the best known are lipopolysaccharide-binding proteins (LBP). The LPS-LBP complex is recognized by the CD14 co-receptors that are present in the cell membrane of immune cells such as macrophages, monocytes and neutrophils. sCD14 is a carrier protein found in soluble form, which allows the activation of other cells that do not have CD14 in their cell membrane, such as endothelial and epithelial cells, dendritic cells, fibroblasts and smooth muscle cells.
The LPS-LBP-CD14 or sCD14 complex is recognized by the Toll-like receptor 4 (TLR4), present in the membrane of myocytes, adipocytes, immune and epithelial cells. CD14 then reacts with the TLR4 on the cell membrane, which, in turn, binds to the MD-2 protein and generates an intracellular signal that stimulates the inflammatory response. The signal activates nuclear factor-κB (NF-κB), gene transcription, and the production of cytokines, including interleukin 1 and 6 (IL-1 and IL-6) and tumor necrosis factor alpha (TNFα). The released cytokines stimulate chemotaxis (the attraction of other immune cells to the area to strengthen the immune response), and the proliferation and differentiation of leukocytes. TNF can produce apoptosis (programmed cell death). TNFα stimulates the secretion of IL-1 and IL-6 by macrophages, enhancing the response. Interleukins also stimulate the differentiation and proliferation of B cells that produce immunoglobulins (Ig), especially IgM and IgG. Signaling ends with endocytosis and degradation of TLR4 and LPS.
Role of the liver in endotoxin detoxification
The liver is the most important organ involved in the detoxification of endotoxins. The release and presence of cytokines in the blood stimulate hepatocytes in the liver to produce acute phase proteins (APP) such as LBP, haptoglobin (Hp), C-reactive protein (CRP), pig major acute phase protein (pig-MAP) and serum amyloid A (SAA).
LPS can pass into the liver from the blood and lymph, through the portal vein, where they will be detoxified, mainly by the enzymatic action of lipase acyloxyacyl hydrolase (AOAH). AOAH is found in the kupffer cells of the liver, but it is also present in macrophages, dendritic cells, neutrophils and renal cortical tubule cells. LPS can also be excreted from the liver into the duodenum via bile, where it is detoxified by the detergent effect of bile salts in the intestine. Chylomicrons, lipoproteins involved in triglyceride transport, play an important role in binding LPS and facilitating its contact with bile, reducing its toxicity.
LBP can catalyze the transfer of LPS to high-density lipoproteins (HDL) to promote their elimination through the liver. These LPS bound to HDL will be transferred to low-density lipoproteins (LDL) that will bind to the LDL receptors of hepatocytes, producing their endocytosis and elimination. Chylomicron-LPS-LBP complexes are also formed, reducing the toxicity of LPS and facilitating its subsequent recognition and detoxification by LDL and LDL receptors in hepatocytes.
If LPS levels are high, inflammatory cytokines (IL-1 and IL-6) pass from the systemic circulation to the hypothalamus where they stimulate the production of prostaglandins, inducing fever and an increase in circulating neutrophils.
Endotoxin effects
Endotoxins are recognized as a foreign body and activate an immune response. Normal levels of endotoxins will be fought by the piglet and will not induce any clinical signs. However, the immune response requires nutrients and energy that cannot be used for lean growth of the animals, reducing their performance and feed efficiency. Depending on the intensity of the immune response, the released cytokines produce effects on the piglet’s metabolism, shifting to a catabolic state, to generate amino acids and energy necessary to sustain immune cell proliferation, synthesis of APP and fever. At the same time, nutrient digestion and absorption at the intestine will be affected. Cytokines action on endothelial cells causes a local inflammatory reaction characterized by edema of the intestinal submucosa. Edema has the potential to progress to bleeding and necrosis (tissue death). This further compromises tight junctions and their barrier function. The performance penalty can be increased by the reduction in consumption and the increase in cortisol induced by cytokines. Cortisol promotes the degradation of proteins and lipids, further worsening the performance of piglets.
The toxicity produced by endotoxins depends on the type of LPS, the route of entry, its concentration and the duration of exposure. Toxic effects also depend on the species and the individual sensitivity of the animal, and the presence of other infectious diseases that may aggravate the situation.
During the process, other cytokines and enzymes are also released that, together with IL-1 and IL-6, can cause vascular damage and intravascular coagulation. In severe cases, when the detoxification capacity is exceeded, systemic inflammation, septic shock and endotoxemia can occur, which can lead to multiple organ failure and the death of the animal. Sepsis and endotoxemia are characterized by fever, intravascular blood clotting, profound pulmonary hypertension, systemic arterial hypotension, decreased cardiac output, a reflex increase in heart rate, and accelerated breathing.
Clinical symptoms in endotoxemic piglets start with recurrent coughing, excessive salivation and even diarrhea and vomiting. Their condition then deteriorates, manifesting generalized malaise, fever, reluctanc to move and recumbency, showing severe respiratory distress (dyspnea) and a bluish coloration of the skin (cyanosis) until death.
Controlling damage and protecting weaned piglets from endotoxins
Measures to counteract endotoxins include those aimed at reducing the presence of gram-negative bacteria as well as direct measures to inactivate the action of the endotoxins. Measures aimed at reducing contamination by gram-negative bacteria include actions to reduce their environmental burden, such as implementing biosecurity protocols, optimizing cleaning and disinfection practices, adequately ventilating facilities to reduce dust, and ensuring access to drinking water. In addition, all nutritional strategies that prevent intestinal dysbiosis and the proliferation of gram-negative bacteria should be included, such as the use of acids, prebiotics and/or probiotics in feed, increased nutrient digestibility, formulations with adequate levels of amino acids, crude protein, fiber and fermentable carbohydrates, among other essential components.
Furthermore, it is essential to reduce stress factors and adapt the piglet to a solid diet before weaning through an appropriate nutritional program. Using fiber sources that improve intestinal integrity and the use of antioxidants can be other complementary strategies.
Measures to reduce the direct action of endotoxins are very limited. The use of vaccines against endotoxins has been tested with promising results in humans, but their use in animal production is very limited due to their high cost. Another option is the use of endotoxin binders in the GIT. This method follows the same reasoning as mycotoxin binders, widely known in animal production. Endotoxin binders are added to feed and adsorb endotoxin in the GIT, preventing their local action in the gastrointestinal mucosa and their absorption into the bloodstream and systemic effects. The selection of an endotoxin binder product with proven scientific in vitro and in vivo data can be an effective strategy in protecting weaned piglets from endotoxins, at a very reasonable cost.
References available on request